Abstract
Patients diagnosed with secondary AML (sAML; ie, following an antecedent myeloid disease or as a late complication of cytotoxic/radiation therapy) have poorer survival than those diagnosed with de novo AML (Granfeldt Østgård LS, et al. J Clin Oncol . 2015;33:3641-49). CPX-351 is a dual-drug liposomal encapsulation of cytarabine and daunorubicin designed to achieve synergistic antileukemia activity. CPX-351 demonstrated a significant overall survival (OS) benefit versus conventional cytarabine and daunorubicin (7+3) in adults aged 60-75 years with newly diagnosed sAML enrolled in phase 2 and 3 studies (Lancet JE, et al. Blood . 2014;123:3239-46 and Lancet JE, et al. ASCO 2016. Abstr 7000). Here, data for patients with sAML were pooled from these studies to more thoroughly evaluate the efficacy and safety of CPX-351 in this patient population.
Patients were randomized 2:1 in the phase 2 study (NCT00788892) and 1:1 in the phase 3 study (NCT01696084) to receive induction with 1 to 2 cycles of CPX-351 (100 units/m2 [100 mg/m2 cytarabine and 44 mg/m2 daunorubicin] on Days 1, 3, and 5 [Days 1 and 3 for second induction]) or conventional 7+3 (cytarabine 100 mg/m2/day × 7 days [5 days for second induction] + daunorubicin 60 mg/m2 on Days 1-3 [Days 1-2 for second induction]). In the phase 2 study, patients with persistent disease after 1 or 2 induction courses of 7+3 were allowed to cross over to receive CPX-351 as salvage therapy and were censored at this time for OS in these analyses. Patients with complete remission (CR) or CR with incomplete platelet or neutrophil recovery (CRi) in either study could receive up to 2 consolidation cycles. Hematopoietic cell transplantation (HCT) was performed at the investigator's discretion. OS was censored at 1 year or at cross over, as many patients in the phase 2 study had informed consent for only 1 year of follow-up.
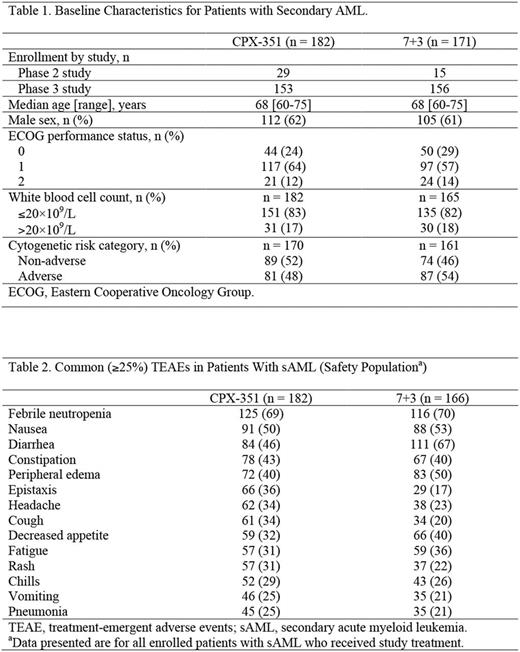
A total of 182 and 171 patients from the CPX-351 and 7+3 cohorts, respectively, were identified as having sAML and were included in the analysis. Baseline characteristics were generally balanced between treatment cohorts (Table 1). When censored at 1 year after the start of treatment or at cross over, patients in the CPX-351 cohort had significantly improved OS (9.63 months) compared with patients in the 7+3 cohort (5.59 months; hazard ratio [HR] = 0.64 [95% CI: 0.49, 0.83]). Kaplan-Meier estimates of OS at 1 year were 42% for CPX-351 and 27% for 7+3. Rates of CR and CR+CRi were higher with CPX-351 than with 7+3 (CR: 36% vs 26%, respectively; odds ratio [OR] = 1.64 [95% CI: 1.040, 2.594]; CR+CRi: 48% and 33%, respectively; OR = 1.92 [95% CI: 1.248, 2.961]). A greater proportion of sAML patients treated with CPX-351 underwent HCT (32%) than those treated with 7+3 (25%; OR = 1.47 [95% CI: 0.924, 2.349]).
Among treated sAML patients, the safety profile of CPX-351 was generally consistent with that of 7+3. The most frequently reported treatment-emergent adverse events (TEAEs) in this combined analysis are shown in Table 2. Grade 3-4 TEAEs were reported for the majority of patients in both treatment cohorts (CPX-351, 91%; 7+3, 90%). Serious TEAEs occurred in 59% and 44% of patients in the CPX-351 and 7+3 cohorts, respectively. The most common (³5%) serious TEAEs were febrile neutropenia (CPX-351, 9% and 7+3, 5%), sepsis (8% and 4%), pneumonia (7% and 5%), respiratory failure (6% and 5%), and ejection fraction decreased (5% and 5%). Few patients discontinued treatment due to a TEAE (2% in each group). In both cohorts, 20% of sAML patients experienced grade 5 TEAEs, including 9% in the CPX-351 cohort and 16% in the 7+3 cohort who died during the treatment period. The most frequent grade 5 TEAEs were disease progression (CPX-351, 4%; 7+3, 4%), sepsis (4%; 2%), and multi-organ failure (1%; 2%). Early mortality rates were lower in sAML patients with CPX-351 than with 7+3 at both Day 30 (5% vs 11%) and Day 60 (12% vs 23%). In both the phase 2 and phase 3 studies, CPX-351 treatment was associated with delayed neutrophil and platelet recovery compared with 7+3.
In this combined analysis, CPX-351 provided significant survival benefit compared with 7+3 in this population of older adults with newly diagnosed sAML and allowed more patients to achieve remission and undergo subsequent HCT.The safety profile of CPX-351 in sAML patients was comparable with that of 7+3 and demonstrated lower 30- and 60-day mortality rates. These data provide further support for CPX-351 as a therapeutic option for older adult patients with sAML.
Lancet: Biopath, Biosight, Boehringer Ingelheim, Celator/Jazz, Celgene, Janssen, Karyopharm Therapeutics, and Novartis: Consultancy; Pfizer: Other: Institutional research funding. Ritchie: Bristol-Myers Squibb: Other: Research funding to my institution; Astellas Pharma: Other: Research funding to my institution; Novartis: Consultancy, Other: Research funding to my institution, and travel, Speakers Bureau; NS Pharma: Other: Research funding to my institution; Pfizer: Consultancy, Other: Research funding to my institution; Incyte: Consultancy, Speakers Bureau; Celgene: Consultancy, Other: Travel, Speakers Bureau. Uy: GlycoMimetics: Consultancy; Boehringer Ingelheim: Consultancy; Novartis: Consultancy, Other: Travel Suppport. Lin: Jazz Pharmaceuticals: Consultancy. Hogge: Novartis, Roche, and Sanofi: Consultancy. Stuart: Incyte: Research Funding; ONO: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Research Funding; Sunesis: Consultancy, Honoraria, Other: Travel Support, Research Funding; Agios: Research Funding; Bayer: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; MedImmune: Research Funding; Celator/Jazz: Research Funding; Novartis: Research Funding; Seattle Genetics: Research Funding; Cantex: Research Funding; Amgen: Consultancy, Honoraria. Strickland: Baxalta: Consultancy; Alexion Pharmaceuticals: Consultancy; Astellas Pharma: Honoraria; Tolero Pharmaceuticals: Consultancy; Sunesis Pharamaceuticals: Consultancy, Research Funding; Boehringer Ingelheim: Consultancy; CTI BioPharma: Consultancy; Daiichi Sankyo: Consultancy. Stone: Ono: Membership on an entity's Board of Directors or advisory committees; Seattle genetics: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Sumitomo: Membership on an entity's Board of Directors or advisory committees; Cornerstone: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Orsenix: Membership on an entity's Board of Directors or advisory committees; Argenix: Other: DSMB; Arog: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Actinium: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees, Research Funding; Fujifilm: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: DSMB. Kolitz: Boehringer Ingelheim, Cantex, Erytech, and Millennium: Research Funding; Gilead, Magellan, Novartis, Pharmacyclics, and Seattle Genetics: Consultancy; Gilead, Novartis, and Seattle Genetics: Other: Travel Support; Gilead, Magellan, and Novartis: Honoraria; Celgene, Jazz: Equity Ownership. Schiller: Celator/Jazz: Research Funding. Wieduwilt: Sigma-Tau: Research Funding; Reata Pharmaceuticals: Equity Ownership. Kovacsovics: Celgene: Consultancy; Flexus: Research Funding; Seattle Genetics: Research Funding. Ryan: Celator/Jazz: Employment, Equity Ownership. Chiarella: Celator/Jazz: Employment, Equity Ownership. Louie: Celator/Jazz: Employment, Equity Ownership, Patents & Royalties. Cortes: ImmunoGen: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Teva: Research Funding; ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Sun Pharma: Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.